Several reasons as follows. This is because sand has a lower heat capacity than water.

What Gets Warmer Sand Or Dirt Lisbdnet Com
The matter that has a higher specific heat capacity takes more joules of energy to raise its.

. We are comparing the heat absorbtion retention and transfer properties of water sand and metal. Sand has a lower specific heat. Why does land heat up faster than water.
Water has a specific heat which requires one calorie of energy to raise the temperature of one gram of water by one degree Celsius. Answer 1 of 10. If none of this heat is conducted away the sand will reach a higher temperature than water because of the lower specific heat.
When put in a hot oven we observed that the metal warmed the fastest and water the slowest. 1 Sand has less specific heat 2 It doesnt mix so easily so the heat basically stays at the surface. A materials specific heat capacity has to do with many factors but one of the most important is the mass of its individual atoms or molecules.
Sand has a higher convection factor which means that heat generated by sunlight landing on a beach is carried away faster in water because the circulation created by the heat hot water rising so the temperature heat concentration is higher on. Sand absorbs radiation almost all of it better than water. Sand particles cannot bathe your hand as thoroughly as water thus the surface area of contact is less and the rate of heat conductance will be lower.
Im assuming youre talking about sand and water under sun light. This is because water has a higher specific heat ca- pacity than sand meaning that it takes a lot of heat or energy to raise the temperature of water one degree whereas it takes comparatively little energy to change the temperature of sand by one degree. The sand should both heat and cool faster than the water.
However the heat does get conducted away. Sand is less dense than water and cools up faster. 8 Why does land heat up faster than water quizlet.
But again since it is conducted away more slowly by the sand than by the. This is because water has a higher specific heat ca- pacity than sand meaning that it takes a lot of heat or energy to raise the temperature of water one degree whereas it takes comparatively little energy to change the temperature of sand by one degree. In other words.
Because the molecules of the sand is compacted together and because the waters molecules moves from one place to another when heated so the heat is spreaded. The sand should both heat and cool faster than the water. Kenzie and Laila Develop and use a model to describe how unequal heating and rotation of the Earth cause patterns of atmospheric and oceanic circulation that determine regional climates.
Sand heats up faster than water because it has a lower specific heat. A material with more surface area per mass will heat and cool faster. O The final temperature of the water in the glass and the copper.
Geometry may play a role in your observation than at room temperature colder than your hand sand feel warmer than water. Does sand melt ice faster than salt. The salt or sugar in an ice cube absorbs the surrounding heat energy faster than frozen water.
The heat capacity of a substance depends on the number of ways that heat energy can be taken up at the particle level- vibrational translational etc other than simple movement of the molecules Kinetic energyThe particles Si O Na etc must have less ways to distribute the radiant energy of the sun and thus it takes less Joules of energy to raise the temperature a. Water will reflect more of radiation. Since it holds a lot less energy it cools down much faster than sand.
O Heat will flow from the water in the glass to the copper until both reach the same temperature. Why does dry soil heat up faster than water. Heat of water is much greater than soil it takes more energy to give the same mass of soil to the same temperature.
It turns out Why do some objects get hot more quickly than othersA materials specific heat capacity has to do with many factors but one of the most important is the mass of its individual atoms or. Sand only requires 290 joules of heat in order for the temperature for 1 gram of heat to increase by 1 degree Celsius. Put more simply the amount of energy it takes to raise a quantity of water by one degree Celsius would raise an equivalent quantity of sand by a little over 14 degrees.
1 See answer Advertisement Advertisement bigr is waiting for your help. Soil has like gaps between the particles which are not good at conducting thermal energy. Water must have 4184 joules of heat for the temperature of 1 gram of water to increase by 1 degree Celsius.
The beach is a perfect example of how much more quickly land heats up than water. First the surface layers of water and sand get heated directly by the sunlight. During the summer sand can become so molten hot its almost unbearable to walk on it.
Cooloing speeds were similar once we took them out of the oven and left to cool at room temperature that is water cooled slowest. Add your answer and earn points. By Staff Writer Last Updated March 27 2020 Sand cools down faster than water because it has a lower specific heat capacity than water.
That is it takes more energy to raise the temperature of water than to raise the temperature of sand by the same amount given equal masses of each substance. Why Does Water Heat Up And Cool Down SlowlyWater heats up or cools down slower than many other common substances because of its high specific heat capacityWhy does water heat and cool slowlyCompared to air or land water is a slow conductor of heat. How Is Sand Warm and Ocean Water Cold.
Likewise sand does not need to lose nearly as much energy as water to produce equivalent cooling. The salt disrupts the equilibrium of water and ice slowing down the amount of water freezing into ice and speeding up the amount of ice melting into water. Because of this the solar power given off by the sun takes a much longer time to take effect and water can get much hotter than regular landforms.
It takes 19 calories per gram to raise the temperature of the sand by 1 degree Celsius. Why does the sand at the beach heat up and cool down faster than the water. Salt melts ice and help prevent re-freezing by.

How Does Specific Heat Of Water Affect Climate Lisbdnet Com
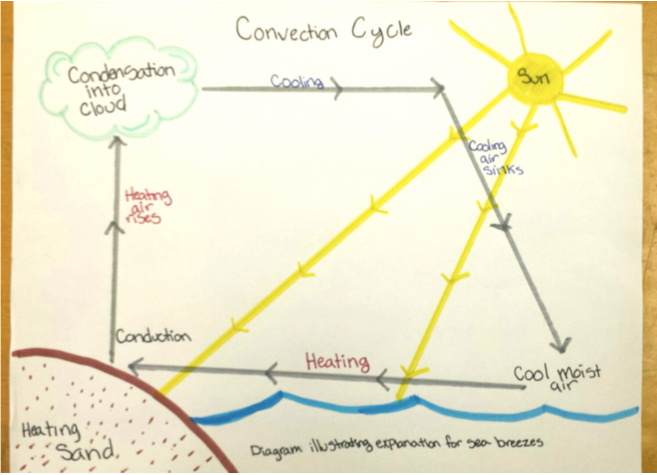
V Using Central Ideas To Explain Intriguing Phenomena Involving Local Weather At The Beach Exploring Physical Phenomena

Specific Heat Of Water Vs Specific Heat Of Sand Science Project Education Com
0 Comments