The compound which consists of two ions that have a great difference in electronegativity is the one with the greatest lattice energy. Lattice energy of an ionic compound depends upon.
What Is Lattice Energy Chemtalk
The energy value can be estimated using the Born-Haber cycle or it can be calculated theoretically with an electrostatic examination of the crystal structure.
. When determining the Lattice Energy we must consider the value of C Z Z- and Ro. Determine which compound has the larger lattice energy. LatexE - frac N_AMzz-e24 pi epsilon_o r_o 1-frac 1nlatex.
The Lattice Energy of Ionic Compounds is directly proportion to the Charge Density. I think it is common practice to list LiF as the largest lattice energy but technically it is the smallest number. The lattice energies of KF KCl KBr and KI follow the order.
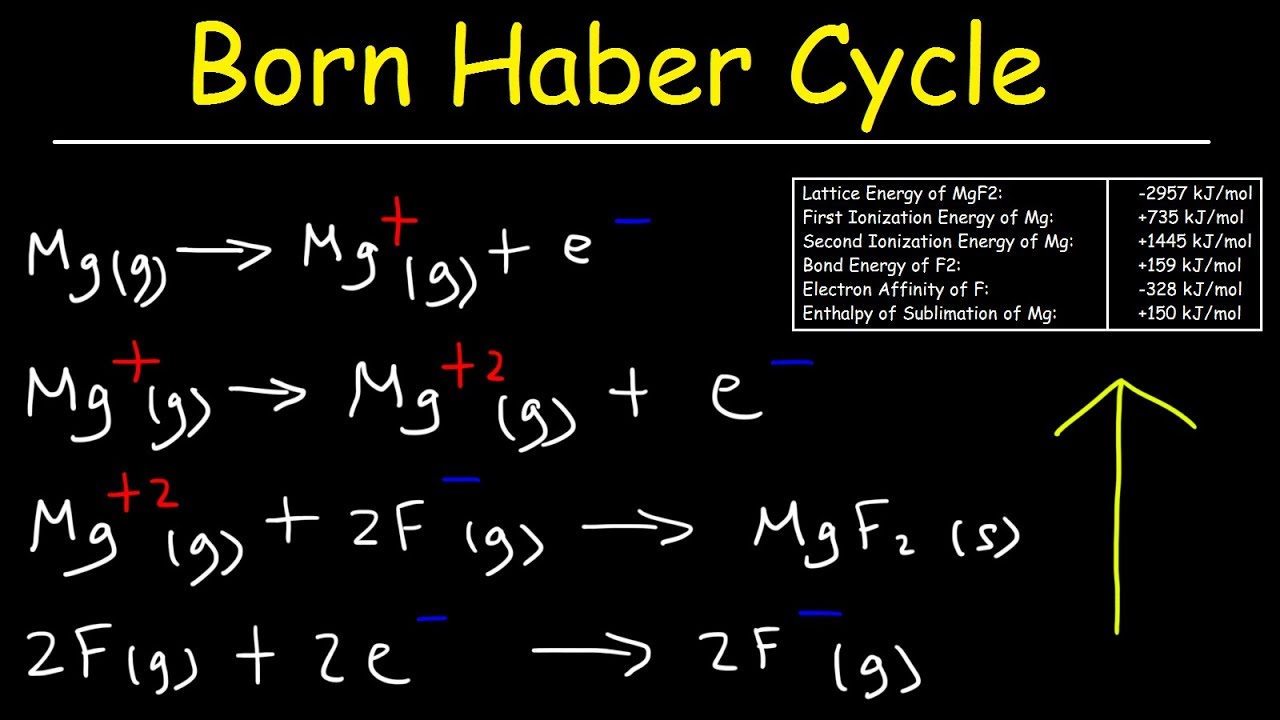
RbI has the largest ions and therefore the weakest lattice energy. Determine the lattice energy for NaCl. Nag Cl-g NaCls Ho -7873 kJmol.
The lattice energies of ionic compounds are relatively large. Increases across a period and decreases down a group. NaCl -787 kJmol.
Thirdly find the ionization energies and electron affinities. Lattice energy decreases as you move down a group. Magnitude of charge on cations and anions.
The closer you are the higher the enter. Lattice energy increases as the magnitude of the charge increases. LiCl -853 kJmol.
Factors Affecting Lattice Energy. I mean so the lattice energy will be higher. M left 1 dfrac191 right 4pt kJ permol endalign.
Using the above formula and known values we have beginalignU_NaCl. Firstly determine the heat of formation. Up to 24 cash back A compound has higher lattice energy if its ions have smaller size and greater charge Li cation is smaller than Mg cation 1 for LiF Fluoride anion is smaller than oxide anion 1 for LiF Mg cation has greater charge than Li cation 1 for MgO Oxide anion has greater charge than fluoride anion 1 for MgO Therefore charge factor favour.
This chemistry video tutorial provides a basic introduction into the lattice energy of ionic compounds. All of these ionic compounds have the same value of C and cations of 1 and anions of 1. Lattice energy of an ionic solid is a measure of the electrostatic force of attraction between oppositely charged ions.
LiF -1036 kJmol. As Ca S have 2 units of charge while K Cl have one and there is not much of a difference in size atleast not enough to overcome the doubling of the charge the charge density of Ca and S ions would be more. The steps to calculate lattice energy based on the Born-Haber Cycle are given below.
What differs in these ionic compounds is the interionic distance gets longer as the ions get larger. From this estimate we can deduce that CaF₂ has the greatest lattice energy. The periodic trend for electronegativity is.
Finally determine the lattice energy by subtracting steps 2 3 from step 1. Chemistry questions and answers. NaF -923 kJmol.
Largest lattice energy MgO 2 and -2 ions with. Magnesium oxide will have the largest lattice energy because it has the largest attraction between the two ions. Consequently the lattice strength of CaS would be more.
In 1918 Born and Lande presented the following model for lattice energy. Which ionic compound has the greatest lattice energy. CaO LiF O NaBr KCI MgO O O O O.
The atomic radius increases as you move down a group. Like any electrostatic force this force is dependent on the charges of the particles and the distance between the charged particles. 1 is a little confusing with the use of the word largest.
1 attempt left Check my work Select the single best answer. Okay because magnesium is a smaller I on and so it will be closer to those bromide ions. Lattice energies are as follows.
The lattice energy ofNaCl for example is 7873 kJmol which is only slightly less than the energy given offwhen natural gas burns. Given that fact the larger the charge on the ions and the smaller the radius of the ions the larger the attraction between them and the more energy required to break them apart lattice energy. The size of the magnesium ion is smaller than the sodium ion.
The higher the distance the lower the lattice energy. Lattice energy dependence on the following factors. The size of the oxide ion though having a larger charge is smaller than chloride because they are in different n shells.
The relation between the magnitudes of lattice energy of crystal and its formation energy is. So in this case magnesium magnesium will have ah higher lattice energy than calcium bromine. Therefore I think you answer to 1 is ok but.
The force of attraction is inversely proportional to the square of the distance so lattice energy decreases as the atomic radius increases. Then find the heat of atomization and dissociation energy. It increases with increase charge on cation.
How to calculate Lattice Energy. Lattice energy is the amount of energy released when.

Lattice Energy Of Ionic Compounds Basic Introduction Charge Vs Ionic Radius Youtube
When Is The Lattice Energy Of An Ionic Compound The Highest Quora

Lattice Energy Of Ionic Compounds Basic Introduction Charge Vs Ionic Radius Youtube
0 Comments